More than 950 people receive first dose of COVID-19 in Vietnam
- First COVID-19 vaccine side-effects reported in Vietnam
- Vietnam to receive nearly 5.6 million COVID-19 vaccine doses
According to the national Expanded Program on Immunization, 433 medical and other frontline workers in Vietnam received the first dose of the AstraZeneca COVID-19 vaccine on August 10, the third day of the first COVID-19 vaccination campaign in Vietnam.
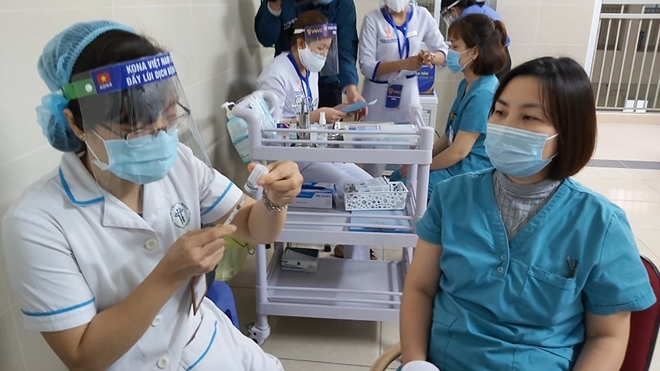 |
| A medical worker receive her first dose of COVID-19 vaccine in Hanoi. |
Up to now, a total of 955 people received the first dose of COVID-19 vaccine in the country, with their health remaining stable, while only some had post-injection common reactions.
Vietnam has begun to roll out its COVID-19 vaccination campaign starting on March 8, prioritized for frontline workers in 13 cities and provinces with recent coronavirus outbreaks. On March 8 and 9, about 522 medical and other frontline workers received the first COVID-19 vaccine dose.
The country has ordered 30 million doses from British-Swedish firm AstraZeneca, and around 117,600 arrived late last month. On March 9, the National Expanded Immunization Program revealed that, Vietnam will receive 5,657,000 doses of COVID-19 vaccine in March and April, all made by AstraZeneca.
Of the amount, 4,177,000 doses will be supplied by COVAX Facility via the UN Children’s Fund (UNICEF), with 1,373,800 doses to arrive on March 25 and another 2,803,200 doses in April.
The AstraZeneca COVID-19 vaccine was co-developed with the University of Oxford and is recommended for people aged between 18 – 64. This vaccine gives high immunity, from 62 – 90% at different doses.
Research shows that all people who were given the vaccine had an immune response to COVID-19 without noticing any problems related to the safety of the vaccine at different doses.
The Ministry of Health is also encouraging all eligible businesses to negotiate with other COVID-19 vaccine suppliers in the world in order to import more vaccines for domestic use in line with the Government Resolution dated February 26, 2021 on the purchase and use of COVID-19 vaccines.

