Vietnam begins human trials of COVID-19 vaccine
Vietnam on December 17 started human trials of a COVID-19 vaccine, with vaccine injections taking place at the Hanoi-based Military Medical Academy. Two men and a woman, in their 20s, whose identities have been kept private, were in stable condition. They will be monitored for 72 hours.
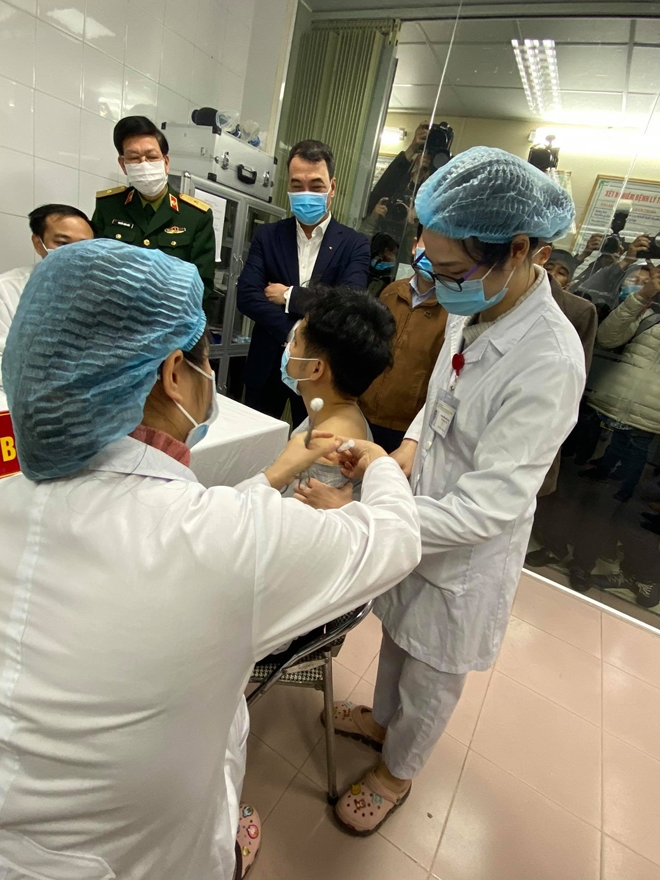 |
| A man is injected with Nanocovax, the first made-in-Vietnam COVID-19. |
The three volunteers were chosen from among more than 200 volunteers, who registered to participate in the human trials of the vaccine, developed by Nanogen Pharmaceutical Biotechnology JSC.
Lieutenant General Do Quyet, director of the university, said: "It is time Vietnam tells the world we can do it, and in fact we have been proving ourselves in the fight against COVID-19 so far."
 |
| Medical officials preparing to give a shot of the COVID-19 vaccine Nanocovax to a volunteer at the Military Medical University. |
Nguyen Ngo Quang, deputy head of the Ministry of Health’s Administration of Science, Technology and Training, said Vietnam "still has a battle against the pandemic ahead, and it needs cooperation from not just scientists but also the authorities and the community, especially volunteers."
But the community should not lower its guard thinking the vaccine is now available and there is no need to continue with the protocols meant to prevent the spread of the virus, he warned. "If the public disregards those protocols, then the efforts of the entire nation will be for nothing."
 |
| Lieutenant General Do Quyet, Director of the Military Medical University checking the preparation for the trial of the country's first COVID-19 vaccine. |
The vaccination on December 17 is part of the first phase of the vaccine trial process in Vietnam, which will have three phases. The first phase of human trials will need 60 people aged between 18 and 50 and in a good health. The selected volunteers have been divided randomly in to 3 groups. In the first phase, all volunteers will receive two intramuscular injections of the vaccine, and the interval of the two injections is 28 days.
The core purpose of the first phase of human trials is to evaluate the safety of a vaccine when used on humans, not its effectiveness, potency, or ability to provoke an immune response in the body.
 |
| A volunteer at the Military Medical University. |
Earlier, on December 10, the National Ethics Council for Biomedical Research under the Ministry of Health approved phase 1 and phase 2 human trials of the Nanocovax COVID-19 vaccine produced by Nanogen in collaboration with the Military Medical University.
 |
 |
| The core purpose of the first phase of human trials is to evaluate the safety of a vaccine when used on humans, not its effectiveness, potency, or ability to provoke an immune response in the body. |
According to the StraitsTimes, Vietnam is among 40 countries in the world that have started human trials of a COVID-19 vaccine, after successfully producing coronavirus test kits early in the pandemic Vietnam has recorded very low coronavirus infections rates, with only 1,405 cases and 35 deaths reported so far.
Apart from Nanocovax, Vietnam has several other COVID-19 candidate vaccines being developed, by Vabiotech, Polyvac, and the Institute of Vaccines and Medical Biologicals.

