Vietnam’s vaccine effective against SARS-CoV-2 variants
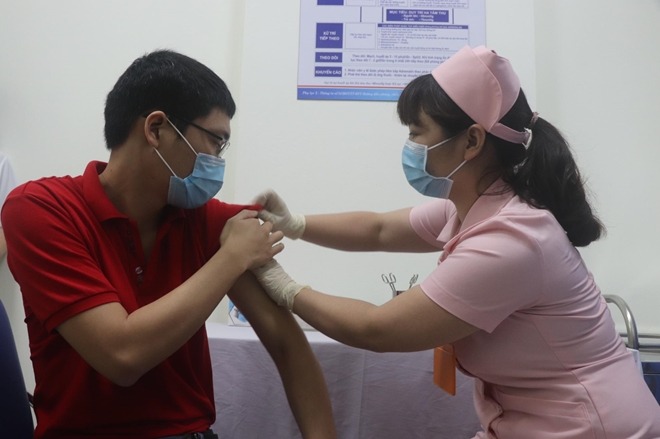 |
| During the vaccination of COVIVAC on a volunteer. |
Human trials on Covivac, the second Vietnam-produced COVID-19 vaccine, started on March 15.
Dr. Duong Huu Thai said that the Covivac vaccine was tested for safety by the National Institute of Hygiene and Epidemiology on March 10.The vaccine has gone through a 7-month research with the support from international partners and under the coordination of the Program for Appropriate Technology in Health (PATH), under the direction of the Government, the Ministry of Health. This vaccine has been sent to domestic competent agencies and foreign countries for evaluation of its safety and immunogenicity as well as its protective effect.
Six out of 120 volunteers have been injected with Vietnam’s second homegrown candidate vaccine COVIVAC, as human trials began at the Hanoi Medical University on March 15.
The 120 healthy volunteers in the first phase have been divided into five groups, including one to receive placebo shots, and will have their health monitored closely for 24 hours after injections.
Each volunteer will have their health checked eight times over a period of 12 months. Following the six injected on March 15, the remainders will take turns being inoculated until April 20. All volunteers will receive their second shots 28 days after the first.
A report on the outcomes of the first phase is expected to be completed in July. If the vaccine meets standards on safety and effectiveness, the second phase will be carried out at the medical center in Thai Binh province’s Vu Thu district with a larger number of volunteers, according to VNA.

