Vietnam to begin human trials of second homegrown COVID-19 vaccine
- VNVC plans to import 30 million COVID-19 vaccine doses
- Vietnam Airlines offers free domestic transportation of COVID-19 vaccines
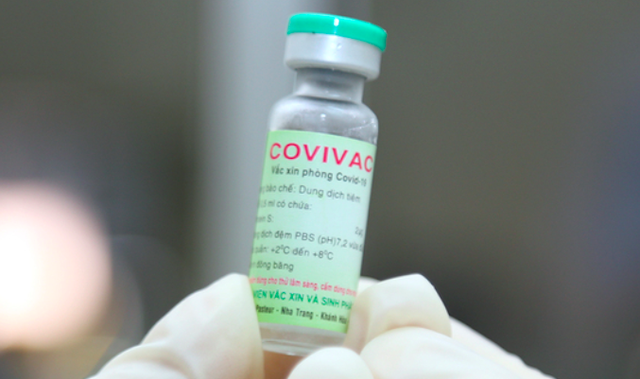 |
| COVIVAC, Vietnam’s second COVID-19 vaccine. |
According to Assoc. Prof. Dr. Vu Dinh Them, Director of the Center for Clinical Trial, six volunteers on will receive the first doses of COVIVAC, Vietnam’s second COVID-19 vaccine to be trialed on humans, at the Center for Clinical Pharmacology of the Hanoi Medical University on March 15.
The research team will monitor the health status of the volunteers. After 8 days, the rest of the 120 eligible volunteers aged 18-50 will also receive the shots.
Volunteers participating in the study will receive two injections (vaccination or placebo injection) with an interval of 28 days. They will be divided into five groups, including one group provided with placebo shots, and their health will be closely monitored within 24 hours after injections.
Each volunteer will have their health checked-up eight times in 12 months.
If the first phase shows positive results and meets safety standards, the second phase will be carried out with a larger sample scale. The first phase is expected to finish in April. The second phase is scheduled to be conducted in Thai Binh province in July this year.
The homegrown vaccine has been developed by the Nha Trang-based Institute of Vaccines and Medical Biologicals (IVAC) and the Hanoi Medical University since last May, using primary chicken embryo cell culture, a technique the institute used previously to successfully produce seasonal flu vaccines.
COVIVAC has undergone pre-clinical trials in India, the US, and Vietnam. The vaccine candidate demonstrated high immunogenicity during pre-clinical trials. It was created based on studies of new SARS-CoV-2 strains.

