Vietnam plans to vaccinate children aged 5 to 11 in 2022
Vietnam will carry out COVID-19 vaccinations for children between the ages of five and 11 next year pending scientific research available, according to the national immunization advisory council.
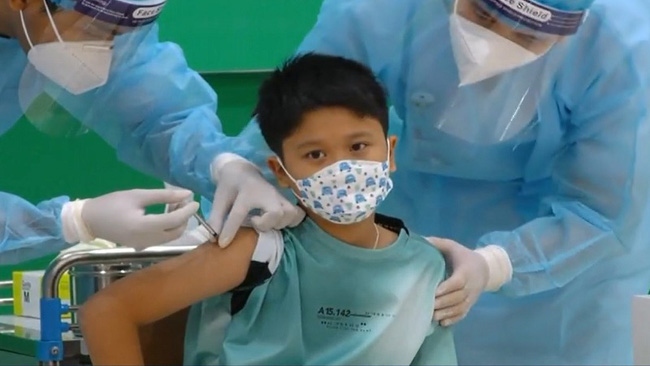
The Ministry of Health has initiated a vaccination plan plan for children in this age group, but the plan must be implemented based on vaccine safety, immunogenicity, and the recommendation of the vaccine manufacturers, said the council.
Amid public disputes over COVID-19 vaccinations for children, Assoc. Prof. & Dr. Tran Minh Dien, director of the Vietnam National Children’s Hospital, said test screening is needed to identify contraindications and prevent complications.
“Children should be vaccinated as part of efforts to reduce severe symptoms in the event that they are infected with the virus, especially in the group of children suffering from underlying diseases, such as cancer, kidney, and liver diseases,” said Dr. Dien. “The vaccine will therefore help children with underlying illnesses reduce their mortality rate as they are in the high-risk group.”
Ho Chi Minh City, the largest coronavirus hotspot in Vietnam, launched its pilot COVID-19 vaccination programme for students aged 16 to 17 on October 27, becoming the first locality nationwide to officially vaccinate children. More than 3,000 students in Cu Chi District and District No.1 were given their first shot.
The city plans to expand the programme to other districts and communes, starting on October 28.
Meanwhile, the national vaccination campaign for children aged 12 to 17 will start in November. The Ministry of Health has approved the use of the Pfizer vaccine (Comirnaty) for the campaign.
Dr. Kidong Park, World Health Organization (WHO) Representative in Vietnam, said extensive vaccination of children over 12 years old will reduce the burden on the health service and lower the risk of new variants emerging, thus potentially reducing viral transmission.
According to Dr. Park, the WHO has granted Emergency Use Listing (EUL) to Pfizer/BioNTech vaccine COVID-19 vaccine for use in adolescents and children above the age of 12.

